.png?width=500&height=750&name=download%20(1).png)
CDC Heeds the Warnings: Spike Protein Persistence Demands Better Monitoring of Blood, Biologics, and Post-COVID Health
A Research Use Only Briefing for Clinicians, Immunologists, and Biologics Manufacturers
Executive Summary
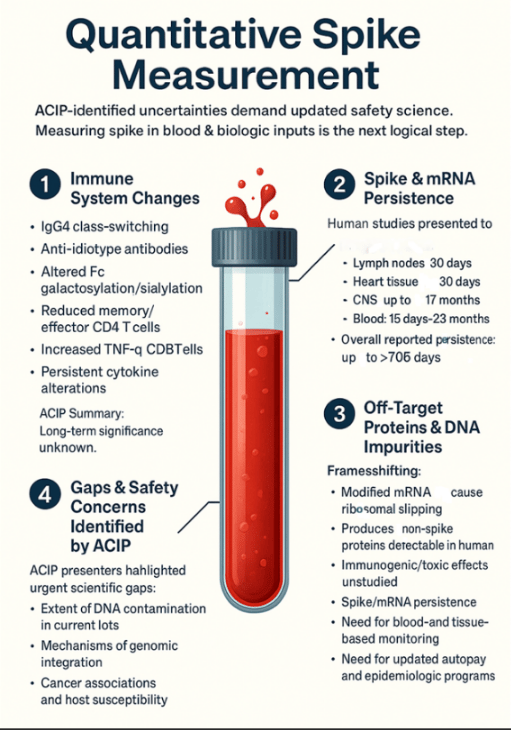
- Researchers (Tufts & Brown) reported vaccine mRNA and spike protein can persist in human tissues—including lymph nodes, heart, CNS, and blood—for up to 706 days, well beyond CDC monitoring windows.
- The same presentation described systemic biodistribution, off-target protein formation via ribosomal frameshifting, and DNA impurities (including SV40 promoter sequences), all with uncertain long-term implications.
- These findings bear directly on blood safety, IVIG, and regenerative biologics, where persistent spike or residual DNA could influence clinical or manufacturing outcomes.
- ACIP recognized a central gap: there are no direct, quantitative tools for measuring spike protein in patients or biologic materials.
- Our quantitative spike assay (RUO) can modernize safety evaluation, replacing assumptions with measurable evidence.
When ACIP’s Briefing Highlighted a Measurement Gap
The CDC’s Advisory Committee on Immunization Practices (ACIP) is known for careful, data-driven discussion—not speculation. Yet at its September 2025 meeting, invited researchers from Tufts and Brown reported findings with profound implications: COVID-19 vaccine mRNA and spike protein can persist in the body, distribute broadly across tissues, generate unintended proteins, and carry biologically active DNA sequences.
Their message was clear: if these materials endure, science must be equipped to measure them—both in clinical contexts and biologics production.
What The CDC Committee Was Shown
Immune change and uncertainty: The briefing detailed immune shifts—including IgG4 class switching, altered Fc patterns, and reduced effector T-cells—with unknown duration or health impact. Presenters noted, “the persistence and potential long-term consequences remain uncertain.”
Systemic biodistribution: Evidence showed mRNA and spike persistence in lymph nodes, heart, blood, and CNS for periods extending from weeks to nearly two years. Notably, these results came from surrogate formulations rather than the final commercial vaccines—yet still demonstrated spread to liver, spleen, brain, and lungs.
Unintended protein synthesis: Modifications like N¹-methylpseudouridine improve mRNA stability but may also cause ribosomal frameshifting, producing off-target proteins detected in human samples. Measuring only spike antibodies is therefore insufficient for safety assessment.
DNA impurities and regulatory blind spots: The briefing cited DNA contamination levels far exceeding FDA limits and identified SV40 promoter sequences in template DNA. No regulatory standards currently address lipid nanoparticle–delivered DNA or its potential biological effects.
Why This Matters for Blood and Biologics
Such persistence and impurities may influence the safety of donor-derived and regenerative biologics. Blood, plasma, cord tissue, or stem-cell–based products could contain unmeasured spike or mRNA, yet no screening mechanisms exist.
Meanwhile, CDC surveillance windows of 0–42 days assume rapid clearance, while ACIP data show persistence approaching two years—suggesting delayed effects could be systematically overlooked.
For manufacturers, these findings emphasize the need for robust quality tools. A spike quantification assay can verify purification, ensure lot consistency, and support donor screening—key steps for advancing transparent, data-backed biologics manufacturing.
A Collaborative Path Forward
The Tufts–Brown presentation underscored an essential truth: good science begins with measurement. Persistence, biodistribution, and off-target effects must move from theoretical debate to quantifiable observation.
To achieve that, we invite clinicians, blood and tissue banks, biologics developers, and research institutions to collaborate through the Spike Clarity Research Network. By combining expertise, we can quantify spike across patient groups, analyze biologic inputs, and build a new framework for safety science in the biologics era.
• Retrospective and prospective safety research
Track spike levels in populations experiencing delayed inflammatory or hematological effects.
• Blood and biologics product characterization
Test donor pools, IVIG lots, and cell-derived therapeutics for spike burden.
• Donor selection optimization
Identify low-spike cohorts for high-purity biologic manufacturing.
• Clinical management of long COVID and spikeopathy
Correlate spike levels with phenotypes and treatment response.
• Regenerative medicine QA modernization
Support standardization—now recognized as essential by leading regenerative medicine conferences.
Conclusion: Imperative to Act Now for Blood and Biologics Safety
For years, discussions about persistent spike or mRNA have been politically charged. But CDC's own proceedings have reframed the issue: this is a scientific and safety question, not a political one.
We now have:
- a plausible mechanism of harm (persistent spike),
- a recognized safety gap (surveillance windows too short),
- and a tool ready for RUO deployment (quantitative spike assay).
The next generation of safety monitoring for blood, tissue, and biologics begins with measurement.
Call to Collaboration: Build the Safety Science ACIP Called For
We invite:
-
- long COVID clinics
- blood and tissue banks
- regenerative medicine labs
- biologics manufacturers
- independent investigators
…to join the Spike Clarity Research Network NOW.
Measurement is how science moves forward. You can help lead that movement.

